The work group currently consists of a total of 7 employees. Main focus is the conduction of preclinical studies regarding implant associated research questions, predominantly in the orthopedic field. Evaluation methods include clinical parameters (X-ray, MRI, CT/µCT), (bio-) mechanics (collaboration with the Laboratory for Biomechanics and Biomaterials, LBB), as well as specialized histological techniques (hard tissue sections, cutting-grinding technique, immunohistochemistry, and others).
Orthopedic implants
Orthopedic implants are routinely used in clinics, e.g. in treatment of bone fractures, malalignment or arthroplasties. Nevertheless, difficulties can occur, like foreign body reactions against the implant, osteolytic processes due to stress shielding effects or implant-associated infections. These and other complications can lead to the necessity of implant removal, which is associated with a high patient burden. Further applications of implants are tendon and ligament ruptures. Due to tissue damage or degenerative processes especially in chronic defects they are often associated with loss of tissue which impedes refixation without bridging implants.
Research foci: degradable implants for tendon and bone application and implant-associated infections
The research group “biodegradable and bioactive implants” deals with three major foci:
The first is the development of dissolving („biodegradable“) implant materials to supersede a second surgery for implant removal. Here, on the one hand, implants for tendon replacement with their special challenges regarding their position at the enthesis are part of the studies. On the other hand, metal based degradable implants for application at the bone site are developed with focus on sufficient strengths for weight bearing applications and slow degradation rates.
Furthermore, development of new concepts for better tissue ingrowth of materials and prevention of implant infections by testing modified (bioactive) implant surfaces are important working points.
For all foci, key questions of biomechanical stability and biocompatibility are examined. Research goal is the development of optimized orthopedic implants and therewith an increased patient safety and quality of life.
Multiple projects, funded by different funding agencies like German Research Foundation (key project in the beginning was the collaborative research center 599 'Sustainable bioresorbable and permanent implants of metallic and ceramic materials'), charitable trusts or economical sponsors resulted in a broad spectrum of established and validated methods for implant research and development with special focus on preclinical testing procedures concerning biocompatibility and functionality.
Used methods in this context are in vitro test systems for the evaluation of degradation kinetics or cell-culture experiments to estimate cytotoxicity in a first step.
Required stability of implants is tested by biomechanical testing procedures like tensile and bending testing as well as cyclic testing. In the further process of preclinical testing, a variety of different specific animal models are established. For detailed quantitative evaluation, for example of implant degradation or bony integration at different time points after surgery, in vivo and ex vivo imaging like radiology, µ-computed tomography, fluorescence imaging or magnetic resonance imaging is used in cooperation with different partners (e.g. Institute of Laboratory Animal Science, Clinic for Laryngology, Rhinology and Otology, both Hannover Medical School or the Veterinary University of Hannover).
In addition, a variety of histological methods is available. Besides different staining techniques including fluorescence microscopy, confocal laser scanning microscopy is used in collaboration with the Research Core Unit for Laser Microscopy of MHH and the Clinic for Prosthetic Dentistry and Biomedical Materials Science here at NIFE for implant surface examination.

The development of implants for tendon repair is conducted within the framework of a DFG-funded research unit (FOR 2180 - tendon and bone junctions) in close interdisciplinary co-working with the Leibniz University of Hannover and the Helmholtz Institute Braunschweig. Focus is the validation of the in vitro results using application-related preclinical animal models which enable the evaluation of the developed implants under due consideration of the whole organism.
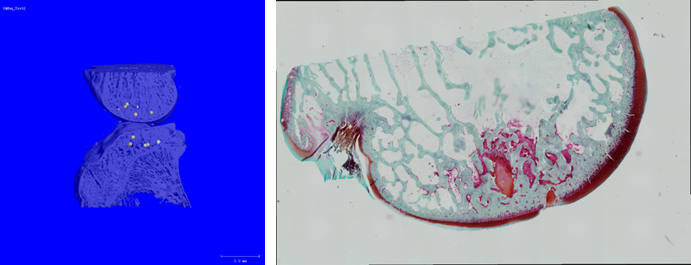
Degradable implant materials are not only advantageous in the common field of fracture healing, but also be transferred to other applications using their property to induce bone increment. Thus, the influence of magnesium based beads on the subchondral bone plate of the knee is currently examined to slow down progression of degenerative cartilage changes during osteoarthritis.
In the research area of implant-associated infections, prophylactic and therapeutic approaches are tested. A new therapeutic strategy, a system called „magnetic drug targeting“ is adapted for implant materials. Herein, special magnetizable nanoparticles are loaded with chemotherapeutics and accumulated magnetically at the desired location. The advantage shall be a reduction of undesired side effects of medication and a better effectiveness by accumulation at the implant surface and in the direct implant interface. Especially the possibility for treatment at any desired time point after implantation with adequate drug levels around the implant is a promising option which might avoid removal of infected implants.

Current projects:



Recent Publications:
Angrisani N, Willbold E, Kampmann A, Derksen A, Reifenrath J.
Histology of tendon and enthesis - suitable techniques for specific research questions.
Eur Cell Mater. 2022 May 24;43:228-251. doi: 10.22203/eCM.v043a16.
Angrisani N, Willumeit-Römer R, Windhagen H, Mavila Chathoth B, Scheper V, Wiese B, Helmholz H, Reifenrath J.
Small-sized magnesium cylinders influence subchondral bone quality in osteoarthritic rabbits - an in vivo pilot study.
Eur Cell Mater. 2021 Sep 28;42:179-195. doi: 10.22203/eCM.v042a14.
Floerkemeier T, Budde S, Willbold E, Schwarze M, Niehof M, Lichtinghagen R, Windhagen H, Weizbauer A, Reifenrath J.
Do biomarkers allow a differentiation between osteonecrosis of the femoral head and osteoarthritis of the hip? - a biochemical, histological and gene expression analysis.
Osteoarthritis Cartilage. 2021 Nov;29(11):1614-1623. doi: 10.1016/j.joca.2021.08.006. Epub 2021 Aug 26.
J, Janßen HC, Warwas DP, Kietzmann M, Behrens P, Willbold E, Fedchenko M, Angrisani N. Implant-based direction of magnetic nanoporous silica nanoparticles - influence of macrophage depletion and infection. Nanomedicine. 2020 Aug 26;30:102289. doi: 10.1016/j.nano.2020.102289.
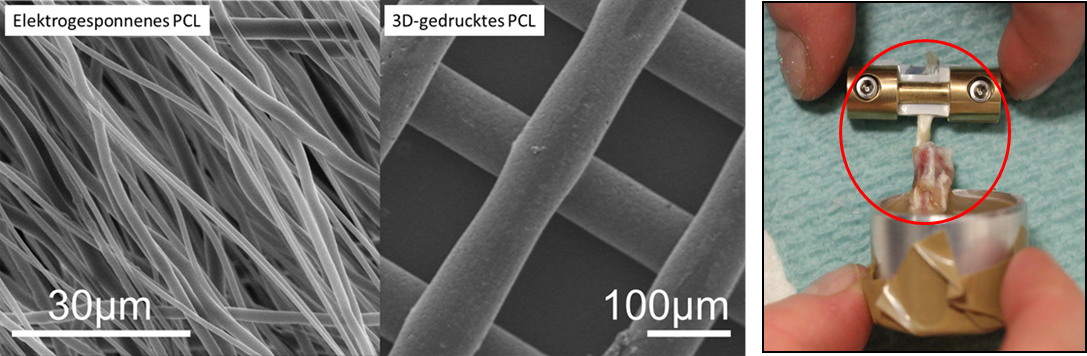
Willbold E, Wellmann M, Welke B, Angrisani N, Gniesmer S, Kampmann A, Hoffmann A, de Cassan D, Menzel H, Hoheisel AL, Glasmacher B, Reifenrath J. Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair. J Tissue Eng Regen Med. 2020 Jan;14(1):186-197. doi: 10.1002/term.2985.
Schröder ML, Angrisani N, Fadeeva E, Hegermann J, Reifenrath J. Laser-structured spike surface shows great bone integrative properties despite infection in vivo. Mater Sci Eng C Mater Biol Appl 2020 Apr;109:110573. doi: 10.1016/j.msec.2019.110573.
Reifenrath J, Wellmann M, Kempfert M, Angrisani N, Welke B, Gniesmer S, Kampmann A, Menzel H, Willbold E. TGF-β3 Loaded Electrospun Polycaprolactone Fibre Scaffolds for Rotator Cuff Tear Repair: An in Vivo Study in Rats. Int J Mol Sci. 2020 Feb 5;21(3):1046. doi: 10.3390/ijms21031046.
Janßen HC, Angrisani N, Kalies S, Hansmann F, Kietzmann M, Warwas DP, Behrens P, Reifenrath J. Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carriers in orthopedics. J Nanobiotechnology. 2020 Jan 15;18(1):14. doi: 10.1186/s12951-020-0578-8.

PD Dr. med. vet. Janin Reifenrath
+49 511 532 8961
Reifenrath.janin(at)mh-hannover.de
NIFE
Stadtfelddamm 34
30625 Hanover
Current projects: